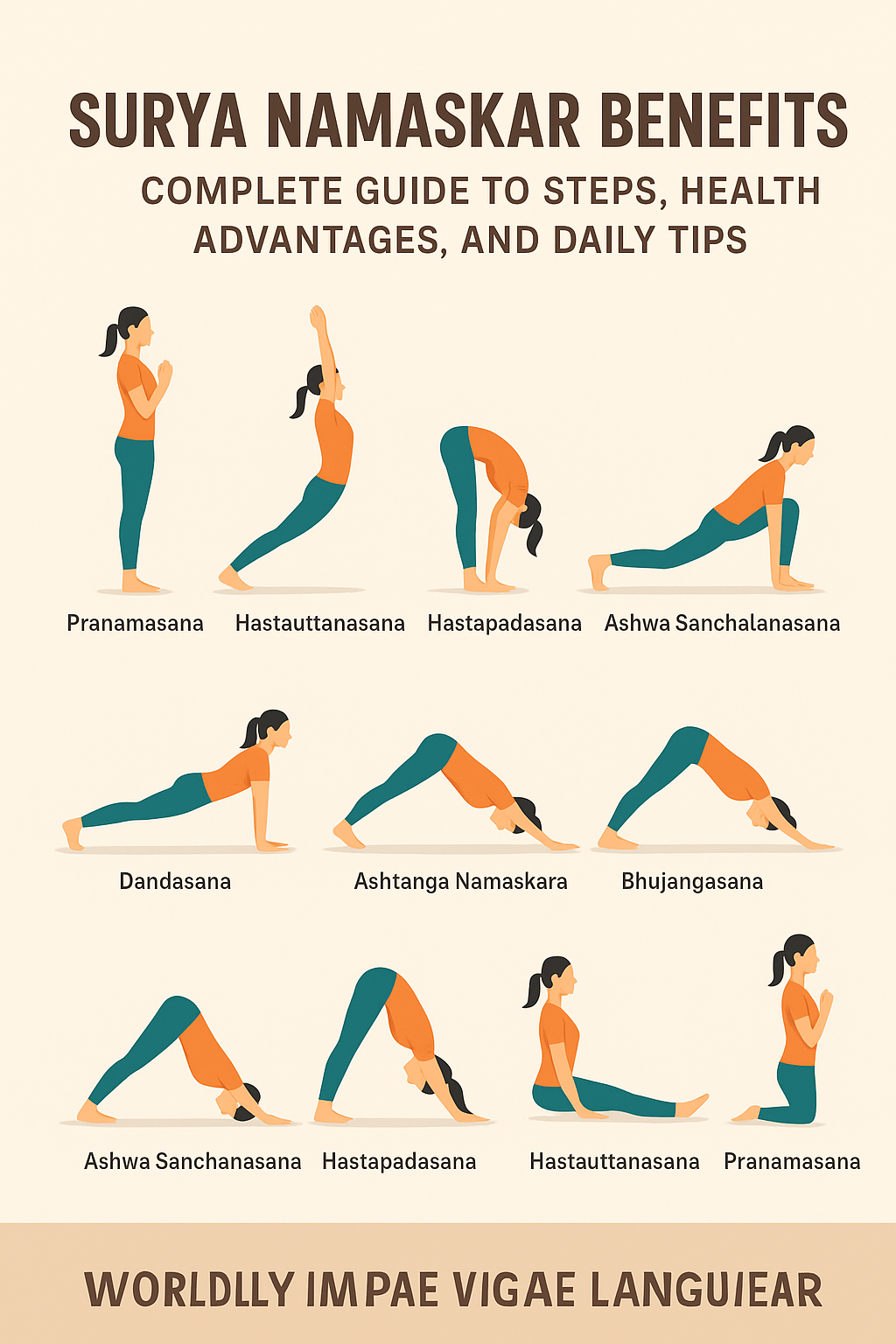
AI in Healthcare is transforming diagnosis, treatment, and operations—discover what it is, how it works, and why it’s redefining modern medicine.
Introduction: What is “AI in Healthcare,” and why does it matter?
AI in Healthcare refers to using machine learning, deep learning, and generative AI to analyze medical data, guide clinical decisions, automate workflows, and personalize patient care. Synonyms you’ll see in this article include medical AI, healthcare AI, and AI-powered medicine. The promise is real and immediate: AI can catch diseases earlier, streamline operations, and support clinicians with evidence-based insights—at scale. The momentum is also measurable. Regulators are authorizing more AI tools than ever, global agencies are issuing governance guidance, and health systems are moving pilots into production. For example, the U.S. FDA’s public list shows over a thousand AI/ML-enabled medical devices authorized to date, reflecting rapid adoption across specialties from radiology to cardiology. U.S. Food and Drug AdministrationSTATNature
How AI in Healthcare works (in plain English)
At its core, AI learns patterns from data. In healthcare, that data can be medical images, EHR notes, lab values, waveforms (like ECGs), genomics, or real-time signals from wearables. Algorithms then:
- Classify (e.g., “Is this tumor malignant?”)
- Predict (e.g., “Who is at high risk of readmission?”)
- Generate (e.g., draft clinical notes or patient instructions with generative AI)
- Recommend (e.g., “Which imaging follow-up is appropriate?”)
Modern deployments combine these capabilities inside clinical workflows (PACS, EHR, RPM dashboards), with governance guardrails guided by frameworks such as the NIST AI Risk Management Framework and its generative AI profile. NISTNIST Publications
Why AI in Healthcare is taking off now
- Regulatory clarity is improving. The FDA maintains a public list of AI/ML-enabled devices; the EMA has published analyses and reflections on AI across the medicines lifecycle. These moves don’t remove all uncertainty, but they signal maturing pathways from R&D to clinical practice. U.S. Food and Drug AdministrationEuropean Medicines Agency (EMA)BioSlice Blog
- Generative AI has crossed a usability threshold. WHO’s 2024 guidance on large multimodal models (LMMs) highlights both powerful use cases and the need for responsible use. World Health Organization+1
- Economic pressure to do more with less. Multiple analyses suggest AI-enabled efficiency can reduce costs and improve experiences when properly implemented, with meaningful savings potential across payers, providers, and hospitals. McKinsey & Company
Today’s high-impact applications
1) Early detection and diagnosis
- Medical imaging: AI triage flags urgent scans (e.g., suspected stroke or pneumothorax) and supports detection of subtle findings. The sheer number of FDA-authorized AI devices—with radiology a leading category—illustrates clinical penetration. U.S. Food and Drug AdministrationNature
- Pathology & dermatology: Pattern recognition helps screen slides and skin lesions; algorithms assist rather than replace clinicians, increasing throughput and consistency.
- Cardiology & neurology: Signal analysis from ECGs and EEGs augments risk prediction and event detection.
Suggested image: “AI reads chest X-ray in ER triage”
Alt: AI in Healthcare chest X-ray triage improves diagnostic speed
2) Personalized and precision medicine
AI integrates genomic, phenotypic, and lifestyle data to tailor therapies—deciding which treatment works for whom, when, and at what dose. Oncology leads (companion diagnostics; response prediction), with fast-following work in autoimmune and rare diseases.
Suggested internal link: Your Guide to Precision Medicine
Suggested image: “Genomic data pipeline feeding AI treatment recommender”
Alt: medical AI for personalized treatment planning
3) Drug discovery and development
From target identification to de-novo molecule generation and trial optimization, AI compresses timelines and boosts hit rates. EMA’s 2024 report catalogs AI use across the medicines lifecycle, underscoring regulators’ growing focus on trustworthy methods and documentation. European Medicines Agency (EMA)
Suggested internal link: Pharma R&D and AI: A Beginner’s Roadmap
4) Virtual care, telemedicine, and remote patient monitoring (RPM)
AI fuses signals from wearables, home blood pressure cuffs, glucometers, and symptom check-ins to detect deterioration early and nudge timely interventions. When combined with telemedicine, this reduces avoidable ER visits and improves chronic disease control.
Suggested internal link: Telemedicine Best Practices for Clinics
Suggested image: “Wearable and smartphone streaming vitals to clinic dashboard”
Alt: healthcare AI monitoring via wearables and telehealth
5) Administrative automation and revenue cycle
Ambient scribing drafts clinical notes; prior-auth and coding tools reduce paperwork; scheduling AI aligns staffing with demand. Analyses project net savings opportunities across payers and providers when adoption is done responsibly. McKinsey & Company
Suggested internal link: EHR Optimization Checklist
Evidence the trend is real (latest data)
- Authorizations: Independent analyses and FDA’s public tracker indicate 1,000+ FDA authorizations for AI/ML-enabled medical devices, with radiology dominating but growth in gastroenterology, cardiology, ophthalmology, and more. A 2025 peer-reviewed review categorized 1,016 authorizations to map clinical and AI features. STATU.S. Food and Drug AdministrationNature
- Policy & guidance:
- WHO (Jan 2024): Guidance for LMMs in health, offering >40 recommendations on safety, efficacy, and rights. World Health Organization
- NIST AI RMF and Generative AI Profile (2024): A voluntary framework that many health organizations use to structure AI risk management. NISTNIST Publications
- EMA (2024): Short report and reflection on AI in the medicines lifecycle—from discovery to post-authorization—highlighting opportunities and regulatory challenges. European Medicines Agency (EMA)BioSlice Blog
Suggested image: “Timeline of AI health policy milestones 2021–2025”
Alt: AI in healthcare policy and governance timeline
Benefits: Why AI in Healthcare improves outcomes and experience
- Faster, more accurate decisions: AI pre-reads images, queues urgent cases, and surfaces relevant history, helping clinicians focus where it matters.
- Capacity expansion without burnout: Automation reduces documentation time and offloads repetitive tasks.
- Proactive, preventive care: Predictive models identify risks before crises—especially useful for chronic and cardiovascular diseases.
- Access and equity: With telehealth and AI triage, underserved regions can get earlier guidance and quicker escalation.
- Operational and financial resilience: When combined with process redesign and change management, organizations can realize measurable cost and productivity gains. McKinsey & Company
Risks & challenges—and how to manage them responsibly
- Data privacy & security: Compliance with HIPAA/GDPR equivalents is necessary but not sufficient. Follow privacy-by-design, minimize data, and encrypt at rest/in transit.
- Bias & fairness: Audit training data; test across demographic subgroups; monitor performance drift. WHO’s 2024 guidance and NIST’s AI RMF outline governance, human oversight, and transparency practices. World Health OrganizationNIST
- Clinical safety & reliability: Validate externally, publish performance, and implement post-market monitoring—consistent with regulator expectations (FDA, EMA). U.S. Food and Drug AdministrationEuropean Medicines Agency (EMA)
- Change management: Tools fail when workflows aren’t re-engineered. Invest in clinician training, feedback loops, and measurable outcome targets.
- Over-reliance on automation: Keep a “human-in-the-loop” and fail-safes (e.g., conservative thresholds).
Outbound link (governance): See the NIST AI Risk Management Framework for a neutral, widely-used structure for identifying, measuring, and mitigating AI risks. NIST
Real-world examples across the care continuum
- Radiology triage & detection: AI flags suspected intracranial hemorrhage on CT scans, moving these to the top of the worklist and prompting faster intervention. The high volume of radiology clearances on the FDA list underscores practical utility. U.S. Food and Drug Administration
- Endoscopy assistance: Computer-aided polyp detection supports gastroenterologists during colonoscopy, improving adenoma detection rates. (Recent FDA authorizations include GI tools such as SKOUT.) U.S. Food and Drug Administration
- Ophthalmology screening: AI detects diabetic retinopathy in primary care clinics, enabling same-day referrals.
- Note drafting with GenAI: Ambient scribe tools summarize encounters and propose structured notes for clinician review—reducing after-hours charting and improving patient rapport.
- Hospital operations: Predictive bed-flow models and AI scheduling stabilize staffing and reduce wait times, contributing to the savings ranges reported by industry analyses. McKinsey & Company
Suggested image: “Endoscopy AI overlay highlighting polyp”
Alt: AI-assisted detection improves colonoscopy outcomes
Building an AI in Healthcare program: a practical roadmap
- Start with high-value, low-regret use cases
- Imaging triage, ambient scribing, and RPM for specific chronic conditions deliver fast ROI and strong clinician support.
- Assemble a cross-functional governance team
- Clinicians, data scientists, security/privacy, compliance, and patient representatives. Align to NIST AI RMF functions: Govern, Map, Measure, Manage. NIST
- Choose the right data foundation
- Standardize (FHIR), de-identify responsibly, and enable secure model training and evaluation. Track lineage and consent.
- Buy vs. build vs. blend
- Evaluate FDA-authorized solutions where possible; when building, plan for clinical validation, documentation, and monitoring consistent with regulator expectations. U.S. Food and Drug Administration
- Pilot with outcomes in mind
- Define metrics (time-to-diagnosis, readmissions, queue time, patient satisfaction); run A/B or stepped-wedge designs; publish results.
- Scale responsibly
- Continuous monitoring for drift/bias; incident response plans; periodic human factors reassessment. Leverage WHO and EMA guidance for policy alignment. World Health OrganizationEuropean Medicines Agency (EMA)
Suggested internal links:
Future outlook: What’s next for AI in Healthcare?
- Digitally native pathways: From AI-assisted triage to AI-supported discharge, care journeys will be redesigned—not just “AI bolted on.”
- Multimodal and longitudinal models: Combining images, text, labs, and wearables over time will improve prognosis and personalization (with strong privacy and consent management).
- Regulation by design: Expect clearer rules on documentation, transparency, and post-market surveillance, influenced by WHO, NIST, and EMA activities. World Health OrganizationNISTEuropean Medicines Agency (EMA)
- Clinical-grade GenAI: LMMs will move from generic chat to embedded, workflow-aware copilots that retrieve patient-specific context securely and cite sources.
Suggested image: “Smart hospital command center with AI dashboards”
Alt: future of AI in healthcare smart hospital operations
Quick FAQs
Is AI replacing doctors?
No. AI augments clinicians by surfacing insights faster and reducing manual work. Clinician oversight remains essential, and regulations require it.
How do we ensure safety and fairness?
Use a risk management framework (e.g., NIST), conduct subgroup performance tests, validate externally, and monitor continuously. NIST
What about data privacy?
Adopt privacy-by-design practices, de-identify wherever possible, and respect local laws and patient consent. WHO’s guidance on generative AI also stresses rights and safety. World Health Organization
Conclusion
AI in Healthcare is no longer a distant promise—it’s a regulated, validated, and increasingly routine part of care delivery. From earlier detection and personalized therapies to ambient documentation and operational optimization, medical AI is already shaping outcomes today. The “what” (pattern-finding algorithms), the “how” (embedding AI into clinical workflows with strong governance), and the “why” (better outcomes, experience, and efficiency) all converge on a clear takeaway: with responsible adoption guided by frameworks like NIST AI RMF and public-interest guardrails from WHO and EMA, AI can help health systems deliver safer, faster, and more equitable care. NISTWorld Health OrganizationEuropean Medicines Agency (EMA)
Sources for latest data & guidance
- FDA. Artificial Intelligence-Enabled Medical Devices (accessed 2025). U.S. Food and Drug Administration
- STAT. As AI device market grows, FDA’s accounting goes silent (June 2025). STAT
- Nature npj Digital Medicine. How AI is used in FDA-authorized medical devices (2025). Nature
- WHO. AI ethics & governance guidance for large multimodal models (2024). World Health Organization
- NIST. AI Risk Management Framework (2023) and Generative AI Profile (2024). NISTNIST Publications
- EMA. Review of AI/ML applications in the medicines lifecycle (2024). European Medicines Agency (EMA)
- McKinsey. Harnessing AI to reshape consumer experiences in healthcare (2024–2025). McKinsey & Company+1